Do you search for 'how to write a balanced equation with a catalyst'? Here you can find questions and answers about the issue.
Table of contents
- How to write a balanced equation with a catalyst in 2021
- What is catalyst in chemistry
- How to write a catalyst in a chemical equation
- Is a catalyst used up in a reaction
- A catalyst changes the mechanism of the reaction
- What happens to a catalyst in a chemical reaction
- What is a catalyst in a chemical reaction
- What is a catalyst in a chemical equation
How to write a balanced equation with a catalyst in 2021
 This picture representes how to write a balanced equation with a catalyst.
This picture representes how to write a balanced equation with a catalyst.
What is catalyst in chemistry
 This image representes What is catalyst in chemistry.
This image representes What is catalyst in chemistry.
How to write a catalyst in a chemical equation
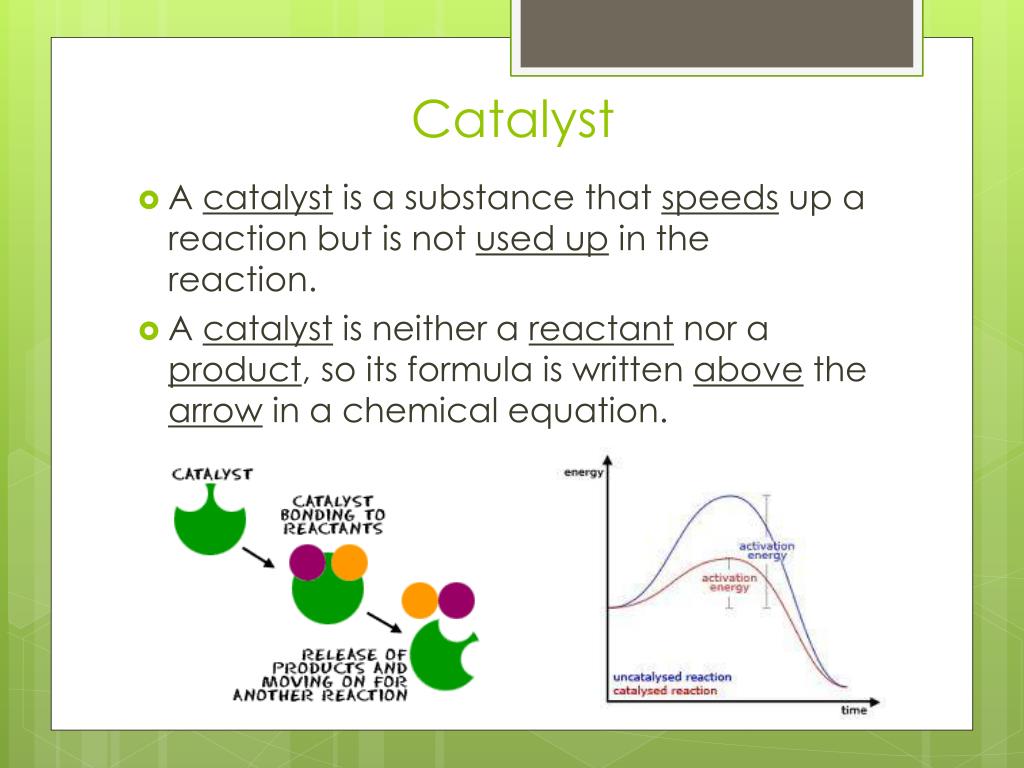 This picture shows How to write a catalyst in a chemical equation.
This picture shows How to write a catalyst in a chemical equation.
Is a catalyst used up in a reaction
 This picture shows Is a catalyst used up in a reaction.
This picture shows Is a catalyst used up in a reaction.
A catalyst changes the mechanism of the reaction
 This image shows A catalyst changes the mechanism of the reaction.
This image shows A catalyst changes the mechanism of the reaction.
What happens to a catalyst in a chemical reaction
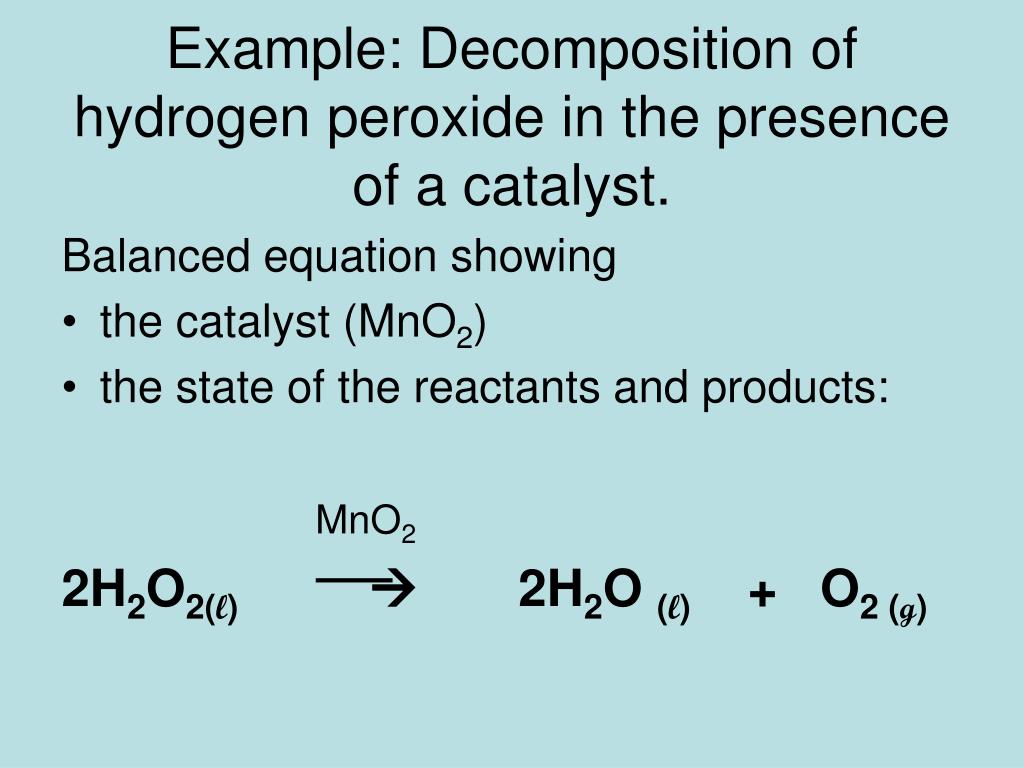 This picture demonstrates What happens to a catalyst in a chemical reaction.
This picture demonstrates What happens to a catalyst in a chemical reaction.
What is a catalyst in a chemical reaction
 This picture illustrates What is a catalyst in a chemical reaction.
This picture illustrates What is a catalyst in a chemical reaction.
What is a catalyst in a chemical equation
 This image representes What is a catalyst in a chemical equation.
This image representes What is a catalyst in a chemical equation.
Which is an example of a balanced equation?
Solved Examples for You 1 HNO 3 +Ca (OH) 2 → Ca (NO 3) 2 + H 2 O 2 BaCl 2 + Al 2 (SO 4 ) 3 → BaSO 4 + AlCl 3 3 Zinc + Silver nitrate → Zinc nitrate + Silver 4 Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
How is the catalyst written in a chemical equation?
The catalyst is often a reactant in an early step in the mechanism and a product in a subsequent step. That makes it appear not to take part in the overall equation for the reaction, when in fact it actually does. Consider the destruction of ozone by a chlorine atom, which is the catalyst. O• and ClO are intermediates.
Why is a catalyst considered in the rate law?
A catalyst is accounted for in the rate law because it speeds up the reaction. It isn't considered in a balanced chemical equation because it doesn't undergo any change, nor does it affect any of the reactants in anyway besides increasing the speed of the reaction. A catalyst is accounted for in the rate law because it speeds up the reaction.
Where are the reaction conditions indicated in a balanced equation?
A lot of times, the reaction conditions such as the temperature, pressure, catalyst etc are indicated above and/or below the arrow in the equation. For example – Question: Write the balanced equations for the following chemical reactions –
Last Update: Oct 2021
Leave a reply
Comments
Selven
21.10.2021 10:13How to balance A chemical reaction away making sure you have the aforesaid number of atoms of each chemical element on both sides. Which one of the following chemical equations is balanced?
Rethal
23.10.2021 10:50Coefficient: a number arranged in front of a formula to balance a chemic equation. We can past find the answer to the equivalence by solving for x.
Ruey
26.10.2021 08:19At present, let's learn how to write A net ionic equation. Equation, and write descending the solution that you fi Nd.